Abstract
Thrombin-stimulated platelets express binding sites for factor VIII (fVIII) despite exposing insufficient phosphatidylserine (PS) to constitute these binding sites. Our previous work implicated soluble fibrin bound to the αIIbβ3 integrin as an important component of these binding sites. Further, we demonstrated that fVIII has direct, high affinity binding to fibrin, with an associated increase in the activity of the purified Xase complex (Gilbert et al, Blood 2015). However, the stoichiometry between fVIII binding sites and fibrin monomers was at least 30:1, suggesting that fVIII binds a minor fibrin(ogen) variant. We undertook experiments to determine the nature of the fVIII-binding fibrin(ogen).
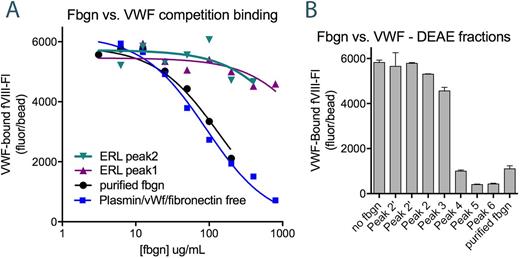
fVIII binding to fibrin(ogen) was evaluated with three complementary assays: i) direct binding of fVIII-fluorescein (fVIII-fluor) to soluble fibrin linked to Superose beads, ii) a competition assay in which binding of fVIII-fluor to fibrinogen competed for binding to Superose-linked von Willebrand factor (VWF) and iii) a factor Xase assay in which fibrin(ogen) enhanced activity. Results were benchmarked against fVIII-fluor binding to thrombin-stimulated platelets.
When fibrinogen (depleted of plasminogen, fibronectin, and VWF) was fractionated by anion exchange chromatography, the fibrinogen γA fraction did not bind fVIII. Rather, the fVIII-binding fractions (constituting 10-15% of total fibrinogen) eluted in association with fibrinogen γ'. However, commercially available γ' fibrinogen does not bind fVIII, suggesting that additional modification is necessary or that fVIII-binding fibrinogen (fbgn*) elutes near fibrinogen γ' on the basis of acidity rather than shared structure. Mass spectroscopy analysis indicated that only traces of VWF remained in the active fractions. Addition of mAb 418, which blocks the fVIII binding site on VWF, had no significant effect on binding of fVIII-fluor to fibrin-Superose showing that residual VWF is not the source of high affinity fVIII binding. Two rabbits inoculated with fbgn*-enriched fractions developed anti-sera that block binding of fVIII to fibrin. In contrast, 5 commercially available polyclonal antibodies against fibrin(ogen) had no effect on fVIII binding, verifying that fbgn* contains a distinct epitope relevant to fVIII binding.
The fbgn*-enriched fraction competed directly with VWF for fVIII binding with at least a 3-fold increase in fVIII binding sites compared to total fibrinogen. Following treatment with thrombin, the fbgn*-enriched fraction had twice as many binding sites for fVIII compared to fibrin from the starting material and a similar affinity in the direct binding assay. FVIII-fluor bound immobilized fibrin with a KD of 2-4 nM while the competition binding experiment yielded an implied KD to fibrinogen of approx. 100 nM. This suggests that thrombin cleavage of fbgn* or assembly into fibrin protofibrils or fibrils increases fVIII affinity approx. 50-fold.
Fbgn* increased activity of the factor Xase complex 2-3 fold. The enhancement was maintained when fbgn* was not exposed to thrombin, indicating that the enhancement was not restricted to the higher affinity binding sites on fibrin.
Plasmin degradation of fibrinogen produces five major fragments that can be separated by ion-exchange chromatography. Enhanced Xase activity and binding of digested fbgn* to fVIII in the VWF competition assay were both similar to intact fbgn*. In order to identify the active fragment, plasmin-digested fbgn* was separated using anion exchange chromatography and tested for factor VIII binding and Xase activity. Only the latest eluting peak bound to fVIII-fluor. SDS-PAGE shows that this peak consists primarily of two bands. IEF gel analysis indicates that the isoelectric points of these two bands differ compared to bands from commercially available γ' digested in the same manner. We are currently determining the identities of the unique bands.
These results show that fVIII binds to a minor, acidic fibrinogen variant (fbgn*) in a manner that competes with VWF binding. Binding to fbgn* enables fVIII binding to thrombin-stimulated platelets and enhances fVIII activity in the Xase complex. Ongoing experiments are expected to identify the molecular features that distinguish fbgn* from fibrinogens γA and γ' and produce selective antibodies that will enable testing of the physiologic contribution of the fVIII-fbgn* interaction to hemostasis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal